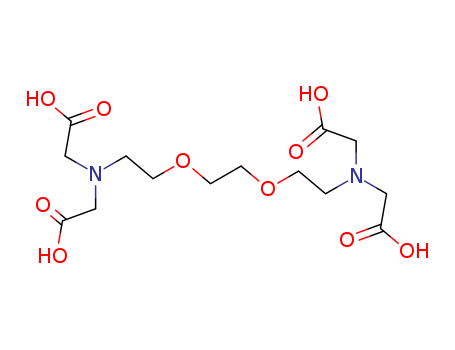
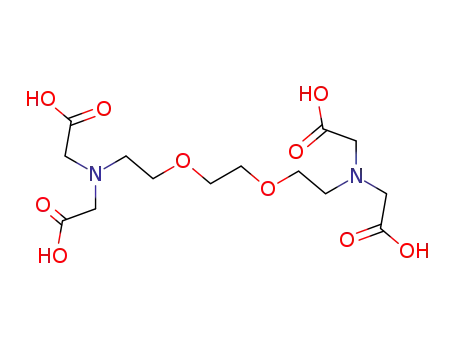
Ethylenebis(oxyethylenenitrilo)tetraacetic acid Good Supplier In Bulk Supply High Purity 67-42-5
- Molecular Formula:C14H24N2O10
- Molecular Weight:380.352
- Appearance/Colour:white to slightly off-white powder
- Vapor Pressure:3.76E-20mmHg at 25°C
- Melting Point:241 °C (dec.)(lit.)
- Refractive Index:1.4590 (estimate)
- Boiling Point:678.016 °C at 760 mmHg
- PKA:1.47±0.10(Predicted)
- Flash Point:363.851 °C
- PSA:174.14000
- Density:1.433 g/cm3
- LogP:-2.03800
Ethylenebis(oxyethylenenitrilo)tetraacetic acid(Cas 67-42-5) Usage
| Description |
Ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) is a diether, with ethylene glycol's hydroxy groups replaced by 2-[bis(carboxymethyl)amino]ethyl groups. |
| Function |
Ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) acts as a calcium chelator, protecting against cell death caused by nitric oxide-induced calcium influx into nerve cells. |
| Comparison with EDTA |
Studies in rats showed that both EGTA and EDTA influence calcium and phosphorus excretion. Unlike EDTA, EGTA does not increase hydroxyproline excretion, suggesting a specific role in calcium chelation. |
| Metal Ion Interaction |
In studies with Euphorbia lathyris, EGTA enhanced biosynthesis of triterpenols and fatty acid esters when used as a metal ion chelator. |
|
Safety Profile
|
Poison by intraperitoneal route. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx. See also GLYCOL ETHERS.
|
|
Purification Methods
|
Dissolve EGTA in aqueous NaOH, precipitate it by adding aqueous HCl, wash it with water and dry at 100o in vacuo.[Beilstein 4 IV 217.]
|
| Toxicity |
Poisonous when administered by intraperitoneal route; moderately toxic if ingested. |
InChI:InChI=1/C14H24N2O10/c17-11(18)7-15(8-12(19)20)1-3-25-5-6-26-4-2-16(9-13(21)22)10-14(23)24/h1-10H2,(H,17,18)(H,19,20)(H,21,22)(H,23,24)/p-2
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
67-42-5 Relevant articles
Effects of ethylenediaminetetraacetate (EDTA) on urinary excretion of hydroxyproline, calcium and phosphorus in the rat
D. N. Kalu, B. C. Miyasaki & G. V. Foster
, Calcified Tissue Research, Volume 16, pages 1–12, (1974)
To determine whether the changes induced by sodium EDTA are due to lowering of plasma calcium, rats were infused with 4.84 mM ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA), a more specific calcium chelator. EGTA increased the urinary excretion of calcium and phosphorus (P<0.001) but not hydroxyproline in thyroparathyroidectomized rats.
Enhancement of Terpenoid Biosynthesis from Mevalonate in a Fraction of the Latex from Euphorbia lathyris
George J. Piazza, James A. Holzwarth
, Plant Physiology, Volume 89, Issue 2, February 1989, Pages 681–686
Conditions for improved incorporation were determined. CaCl2 or CaCl2 plus MnCl2 stimulated biosynthesis, and the metal ion chelator, ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) enhanced stimulation. Ethylenediaminetetraacetic acid was almost as effective as EGTA, but phthalic acid and citric acid were relatively poor stimulators.
67-42-5 Process route
-
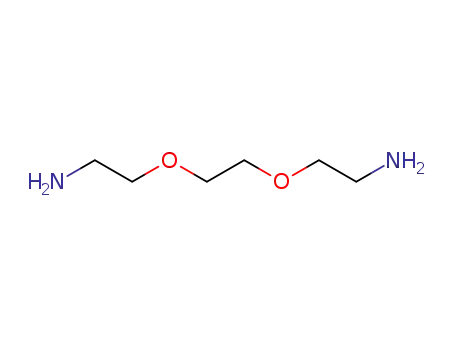
- 929-59-9,892154-56-2
3,6-dioxa-1,8-diaminooctane
-
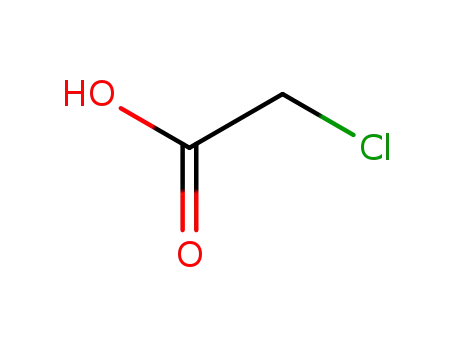
- 79-11-8
chloroacetic acid
-
- 67-42-5
ethylene glycol-bis(2-aminoethyl)-N,N,N'N,'-tetraacetic acid
Conditions
| Conditions |
Yield |
|
With sodium hydroxide;
|
|
-
- 67-42-5
ethylene glycol-bis(2-aminoethyl)-N,N,N'N,'-tetraacetic acid
67-42-5 Upstream products
67-42-5 Downstream products
-
50-00-0
formaldehyd
-
124-38-9
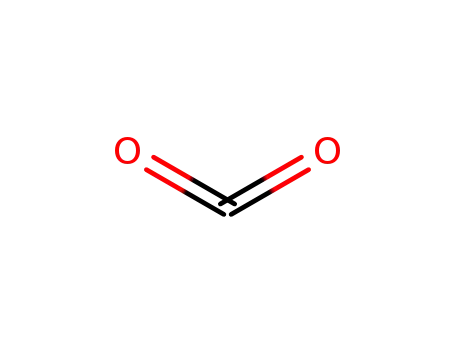
carbon dioxide