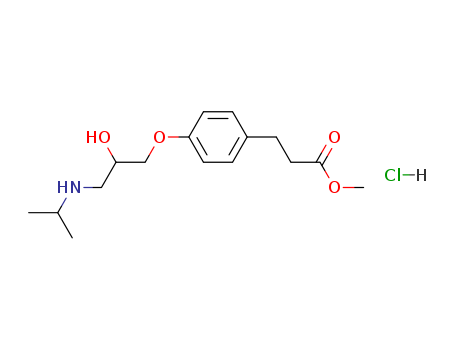
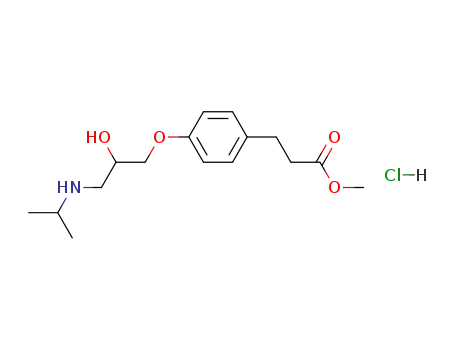
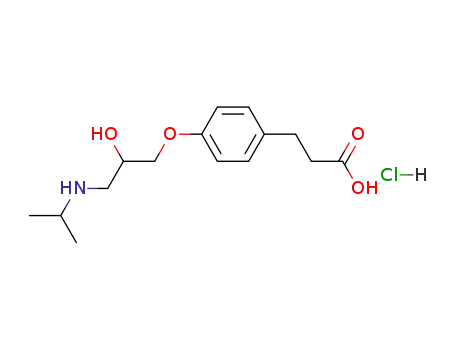
Manufacturer supply Esmolol hydrochloride 81161-17-3 with sufficient stock and high standard
- Molecular Formula:C16H26ClNO4
- Molecular Weight:331.84
- Appearance/Colour:white solid
- Melting Point:48-50 °C
- Boiling Point:430.2 °C at 760 mmHg
- Flash Point:214 °C
- PSA:67.79000
- Density:1.026 g/cm3
- LogP:2.72280
Esmolol hydrochloride(Cas 81161-17-3) Usage
|
Manufacturing Process
|
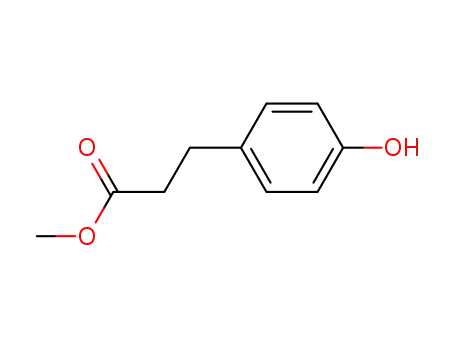
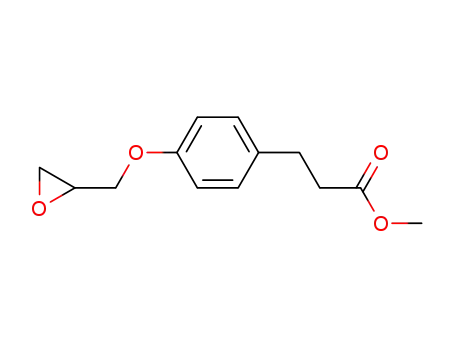
A solution of 17 g (0.1 mole) of 3-(4-hydroxyphenyl)propionic acid in 500 mL methanol and 2 mL concentrated sulfuric acid were placed in a Soxhlet extractor charged with 3A molecular sieves. The solution was refluxed for 72 hours and the sieve were exchanged at 24 hour intervals. The reaction medium was then evaporated to an oil which was dissolved in 100 mL toluene and extracted with 100 mL water (3 times). The toluene phase was dried over magnesium sulfate, treated with activated charcoal and evaporated to provide 15 g (80%) of a clear oil. The NMR spectrum was consistent with the methyl 3-(4-hydroxyphenyl)propionate.The oil described above was utilized directly in the condensation reaction with the epichlorohydrin. A mixture of 0.1 mole of methyl 3-(4- hydroxyphenyl)propionate, 0.2 mole potassium carbonate and 0.4 mole epichlorohydrin in 250 mL acetone was heated to reflux for 24 hours. The reaction medium was then filtered and evaporated. The residue was taken up in 100 mL toluene and washed with 100 mL 1.0 N NaOH and 100 mL water (2 times). The toluene phase was then dried over magnesium sulfate and evaporated to provide the crude product as an oil. Purification was effected by vacuum distillation (156°C/0.4 mm) and provided methyl 3-[4-(2,3- epoxypropoxy)phenyl]propionate. The NMR and IR spectra and elemental analysis data were consistent with the assigned structure.A mixture of 50 g (0.21 mole) of methyl 3-[4-(2,3- epoxypropoxy)phenyl]propionate and 100 mL of isopropylamine in 100 mLmethanol was heated to reflux for 4 hours. The reaction medium was then evaporated and the resulting oil taken up in methanol and treated with ethereal HCl and provided crystals which were recrystallized in similar fashion to provide 28 g (47%) of white crystals: melting point 85-86°C. The NMR and IR spectra and the elemental analysis data were consistent with the structure of methyl 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)benzenepropanoate.In practice it is usually used as hydrochloride.
|
|
Therapeutic Function
|
Beta-adrenergic blocker
|
|
Pharmacokinetics
|
Esmolol Hydrochloride is a beta1-selective (cardioselective) adrenergic receptor blocking agent with rapid onset, a very short duration of action, and no significant intrinsic sympathomimetic or membrane stabilizing activity at therapeutic dosages. Its elimination half-life after intravenous infusion is approximately 9 minutes. Esmolol Hydrochloride inhibits the beta1 receptors located chiefly in cardiac muscle, but this preferential effect is not absolute and at higher doses it begins to inhibit beta2 receptors located chiefly in the bronchial and vascular musculature.Esmolol Hydrochloride is rapidly metabolized by hydrolysis of the ester linkage, chiefly by the esterases in the cytosol of red blood cells and not by plasma cholinesterases or red cell membrane acetylcholinesterase. Total body clearance in man was found to be about 20 L/kg/hr, which is greater than cardiac output; thus the metabolism of Esmolol Hydrochloride is not limited by the rate of blood flow to metabolizing tissues such as the liver or affected by hepatic or renal blood flow. Esmolol Hydrochloride has a rapid distribution half-life of about 2 minutes and an elimination half-life of about 9 minutes.
|
|
Side effects
|
Hypotension (in 20-50% of pts), diaphoresis, pallor, flushing, peripheral ischemia, bradycardia, heart block, heart failure, sudden cardiac death.Dizziness, confusion, headache, fatigue, anxiety.Nausea, vomiting, fever.Bronchospasm, cough, wheeziness, nasal stuffiness.Urinary retention.Inflammation and induration at injection site.
|
|
Veterinary Drugs and Treatments
|
Esmolol may be used as test drug to indicate whether beta-blocker therapy is warranted as an antiarrhythmic agent, particularly in cats with hypertrophic cardiomyopathy, or as an infusion in the shortterm treatment of supraventricular tachyarrhythmias (e.g., atrial fibrillation/flutter, sinus tachycardia).
|
|
Drug interactions
|
Potentially hazardous interactions with other drugsAnaesthetics: enhanced hypotensive effect.Analgesics: NSAIDs antagonise hypotensive effect.Anti-arrhythmics: increased risk of myocardial depression and bradycardia; with amiodarone, increased risk of bradycardia and AV block and myocardial depression; increased risk of myocardial depression and bradycardia with flecainide.Antidepressants: enhanced hypotensive effect with MAOIs.Antimalarials: increased risk of bradycardia with mefloquine.Antipsychotics: enhanced hypotensive effect with phenothiazinesCalcium-channel blockers: increased risk of bradycardia and AV block with diltiazem; severe hypotension and heart failure occasionally with nifedipine and possibly other dihydropyridines; asystole, severe hypotension and heart failure with verapamil - avoid concomitant verapamil use.Antihypertensives: enhanced hypotensive effect; increased risk of withdrawal hypertension with clonidine; increased risk of first dose hypotensive effect with post-synaptic alpha-blockers.Cytotoxics: possible increased risk of bradycardia with crizotinib.Diuretics: enhanced hypotensive effect.Fingolimod: possibly increased risk of bradycardia.Moxisylyte: possible severe postural hypotension.Sympathomimetics: severe hypertension with adrenaline and noradrenaline and possibly dobutamine
|
|
Metabolism
|
Esmolol hydrochloride is metabolised by esterases into an acid metabolite (ASL-8123) and methanol. This occurs through hydrolysis of the ester group by esterases in the red blood cells. Esmolol hydrochloride is excreted by the kidneys, partly unchanged (less than 2% of the administered amount), partly as acid metabolite that has a weak (less than 0.1% of esmolol) beta-blocking activity. The acid metabolite is also excreted in the urine
|
|
Definition
|
ChEBI: The hydrochloride salt of esmolol. A cardioselective and short-acting beta1 receptor blocker with rapid onset but lacking intrinsic sympathomimetic and membrane-stabilising properties, it is used in the management of su raventricular arrhythmias, and for the control of hypertension and tachycardia during surgery.
|
InChI:InChI=1/C16H25NO4.ClH/c1-12(2)17-10-14(18)11-21-15-7-4-13(5-8-15)6-9-16(19)20-3;/h4-5,7-8,12,14,17-18H,6,9-11H2,1-3H3;1H
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
81161-17-3 Relevant articles
Preparation method of iprolol hydrochloride
-
Paragraph 0053; 0057-0059; 0060; 0064-0066, (2021/10/11)
The invention provides a preparation met...
Hitting a soft drug with a hard nucleophile: Preparation of esmolol's metabolite by treatment with bis(tributyltin) oxide
Zhang, Cunyu A.,Erhardt, Paul W.
scheme or table, p. 722 - 726 (2012/01/13)
A facile method for the production of es...
IMAGE-GUIDED THERAPY OF MYOCARDIAL DISEASE: COMPOSITION, MANUFACTURING AND APPLICATIONS
-
Page/Page column 12-13, (2009/10/01)
Compositions and methods for imaging and...
METHOD FOR TREATMENT OR PROPHYLAXIS OF CARDIAC DISORDERS
-
, (2008/06/13)
A method for the treatment of prophylaxi...
81161-17-3 Process route
-
- 5597-50-2
3-(4-hydroxyphenyl)propionic acid methyl ester
-
- 81161-17-3
esmolol hydrochloride
Conditions
| Conditions |
Yield |
|
Multi-step reaction with 2 steps
1: potassium carbonate / acetone / Reflux
2: methanol / Reflux
With potassium carbonate; In methanol; acetone;
|
|
-
- 81147-94-6,246219-23-8
methyl 3-<4-(2,3-epoxypropoxy)phenyl>propionate
-
- 81161-17-3
esmolol hydrochloride
Conditions
| Conditions |
Yield |
|
methyl 3-<4-(2,3-epoxypropoxy)phenyl>propionate; isopropylamine; In methanol; at 50 ℃;
With hydrogenchloride; In methanol; ethyl acetate; at 5 ℃; for 1.5h;
|
95.6% |
|
methyl 3-<4-(2,3-epoxypropoxy)phenyl>propionate; isopropylamine; In methanol; for 4h; Reflux;
With hydrogenchloride; In methanol; diethyl ether;
|
52% |
|
methyl 3-<4-(2,3-epoxypropoxy)phenyl>propionate; isopropylamine; In methanol; Reflux;
With hydrogenchloride; In methanol; diethyl ether;
|
50% |
81161-17-3 Upstream products
-
81147-94-6
methyl 3-<4-(2,3-epoxypropoxy)phenyl>propionate
-
75-31-0
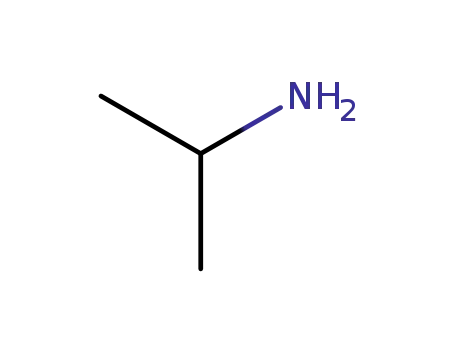
isopropylamine
-
501-97-3
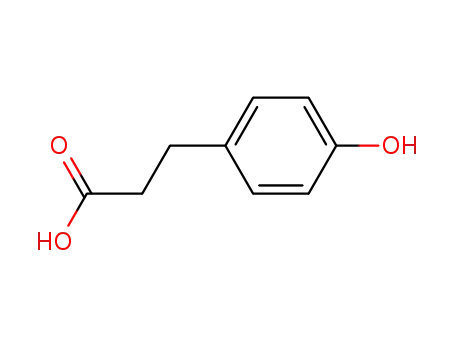
4-hydroxyphenylpropionic acid
-
5597-50-2
3-(4-hydroxyphenyl)propionic acid methyl ester
81161-17-3 Downstream products
-
81148-15-4
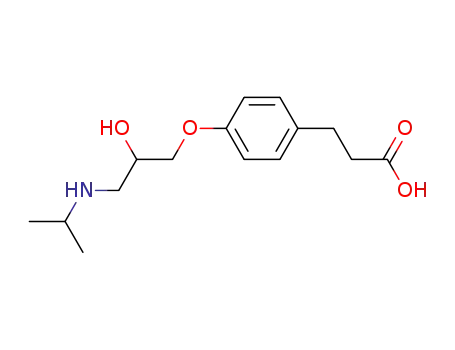
3-<4-<2-hydroxy-3-(isopropylamino)propoxy>phenyl>propionic acid
-
83356-59-6
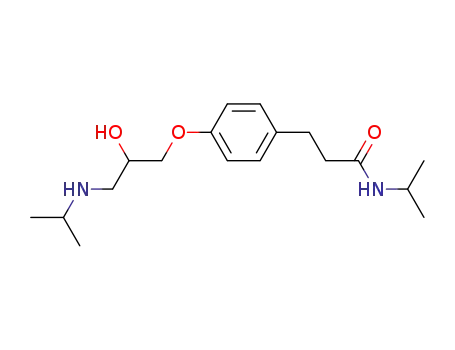
N-isopropyl-3-<4-<2-hydroxy-3-(isopropylamino)propoxy>phenyl>propionamide
-
83356-60-9
3-<4-<2-hydroxy-3-(isopropylamino)propoxy>phenyl>propionic acid hydrochloride