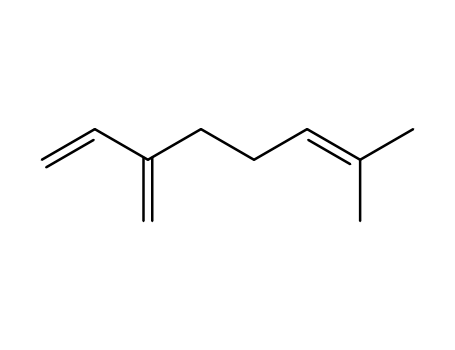
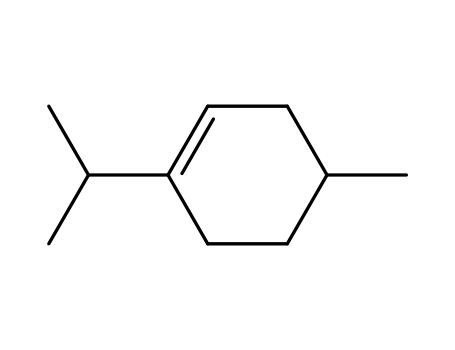
Reliable factory customized supply Myrcene 123-35-3
- Molecular Formula:C10H16
- Molecular Weight:136.237
- Appearance/Colour:clear to pale yellow liquid
- Vapor Pressure:~7 mm Hg ( 20 °C)
- Melting Point:<-10 °C
- Refractive Index:1.4690
- Boiling Point:167 °C at 760 mmHg
- Flash Point:44.4 °C
- PSA:0.00000
- Density:0.769 g/cm3
- LogP:3.47500
Myrcene(Cas 123-35-3) Usage
|
Preparation
|
From linalool
|
|
Definition
|
ChEBI: A monoterpene that is octa-1,6-diene bearing methylene and methyl substituents at positions 3 and 7 respectively.
|
|
Aroma threshold values
|
Aroma characteristics at 10%: terpy, herbaceous, woody with a rosy celery and carrot nuance
|
|
Taste threshold values
|
Taste characteristics at 5 to 100 ppm: woody, vegetative, citrus fruity with a tropical mango and slight, leafy, minty nuance
|
|
Synthesis Reference(s)
|
Journal of the American Chemical Society, 97, p. 3252, 1975 DOI: 10.1021/ja00844a073The Journal of Organic Chemistry, 47, p. 4161, 1982 DOI: 10.1021/jo00142a031Tetrahedron Letters, 25, p. 5193, 1984 DOI: 10.1016/S0040-4039(01)81561-6
|
|
General Description
|
A yellow oily liquid with a pleasant odor. Flash point below 200°F. Insoluble in water and less dense than water.
|
|
Air & Water Reactions
|
Insoluble in water.
|
|
Reactivity Profile
|
The unsaturated aliphatic hydrocarbons, such as MYRCENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions. Many of these compounds undergo autoxidation upon exposure to the air to form explosive peroxides. Violent explosions have occurred at low temperatures in ammonia synthesis gas units. These explosions have been traced to the addition products of dienes and oxides of nitrogen, produced from the interaction of nitrogen oxide and oxygen [Bretherick, 1995].
|
|
Health Hazard
|
May be harmful by inhalation, ingestion or skin absorption.
|
|
Fire Hazard
|
Special Hazards of Combustion Products: Vapor may travel considerable distance to a source of ignition and flashback.
|
|
Safety Profile
|
Low toxicity by ingestion and skin contact. Experimental reproductive effects. A moderate skin and eye irritant. A flammable liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
|
InChI:InChI=1/C10H16/c1-5-10(4)8-6-7-9(2)3/h5,7H,1,4,6,8H2,2-3H3
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
123-35-3 Relevant articles
-
Walker,Hawkins
, p. 4209 (1952)
-
Acid-catalyzed hydrolysis of alcohols and their β-D-glucopyranosides
Skouroumounis, George K.,Sefton, Mark A.
, p. 2033 - 2039 (2000)
The hydrolysis, in model wine at pH 3, o...
Palladium-Catalyzed Reactions of Allylic Electrophiles with Organometallic Reagents. A Regioselective 1,4-Elimination and a Regio- and Stereoselective Reduction of Allylic Derivatives
Matsushita, Hajime,Negishi, Ei-ichi
, p. 4161 - 4165 (1982)
-
-
Yasuda,A. et al.
, p. 1752 - 1756 (1979)
-
Molybdenum- and tungsten-catalyzed allylation of aromatic compounds with allylic esters and alcohols
Shimizu, Isao,Sakamoto, Toshiaki,Kawaragi, Saeko,Maruyama, Yooichiro,Yamamoto, Akio
, p. 137 - 138 (1997)
A novel method to substitute aromatic co...
-
Haley et al.
, p. 264 (1969)
-
Preparation of 1-Alkenes by the Palladium-Catalyzed Hydrogenolysis of Terminal Allylic Carbonates and Acetates with Formic Acid-Triethylamine
Tsuji, Jiro,Minami, Ichiro,Shimizu, Isao
, p. 623 - 627 (1986)
A useful method for the preparation of 1...
Selective Synthesis of 1,3-Dienic Terpenes in a β-Form through Regioselective 1,4-Elimination of Allylic Ethers
Otera, Junzo,Niibo, Yoshihisa,Okuda, Kazuhiro
, p. 1829 - 1832 (1986)
γ-Methyl-substituted allylic ethers prov...
Thermal behaviour of selected C10H16 monoterpenes
Stolle, Achim,Brauns, Claudia,Nuechter, Matthias,Ondruschka, Bernd,Bonrath, Werner,Findeisen, Matthias
, p. 3317 - 3325 (2006)
The presented work investigates the ther...
Flash vacuum thermolysis of terpenic compounds in the pinane series
Lemee,Ratier,Duboudin,Delmond
, p. 1313 - 1318 (1995)
The flash pyrolysis of various derivativ...
-
Tanaka,S. et al.
, p. 3252 - 3254 (1975)
-
-
Liu,R.S.H.,Hammond,G.S.
, p. 1892 - 1893 (1964)
-
-
Hunt,Hawkins
, p. 5618 (1950)
-
Novel route to a fruitful mixture of terpene fragrances in particular phellandrene starting from natural feedstock geraniol using weak acidic boron based catalyst
Eisenacher, Matthias,Beschnitt, Stefan,H?lderich, Wolfgang
, p. 214 - 217 (2012)
Myrcene, ocimene and in particular phell...
Thermal behavior of pinan-2-ol and linalool
Leiner, Janne,Stolle, Achim,Ondruschka, Bernd,Netscher, Thomas,Bonrath, Werner
, p. 8358 - 8375 (2013)
Linalool is an important intermediate fo...
Comparative thermolysis of β-and α-pinenes in supercritical ethanol: The reaction characterization and enantiomeric ratios of products
Chibiryaev,Yermakova,Kozhevnikov,Sal'nikova,Anikeev
, p. 1234 - 1238 (2007)
The thermolysis of β-pinene and the co-t...
Preparation method of myrcene
-
Paragraph 0031-0032; 0041-0042; 0045-0046, (2021/11/19)
The method uses geraniol and/or nerol an...
Method and Means for Releasing a Terpene Mixture to a Cannabis Flower During Storage
-
, (2021/09/17)
A method and means for releasing a terpe...
A donor-acceptor complex enables the synthesis of: E -olefins from alcohols, amines and carboxylic acids
Chen, Kun-Quan,Shen, Jie,Wang, Zhi-Xiang,Chen, Xiang-Yu
, p. 6684 - 6690 (2021/05/31)
Olefins are prevalent substrates and fun...
Method for synthesizing high-purity 13C2-myrcene from 13C2-geraniol
-
Paragraph 0045; 0048, (2020/08/06)
The invention relates to a method for sy...
123-35-3 Process route
-
- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
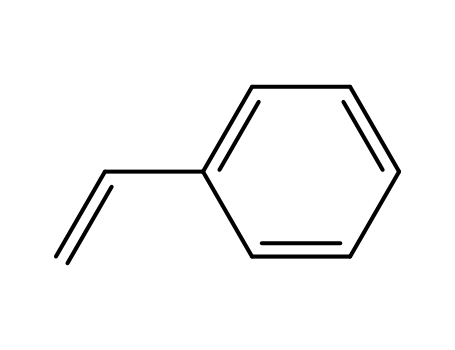
styrene
-
- 563-80-4
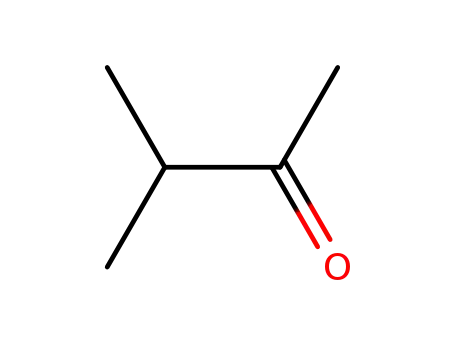
3-methyl-butan-2-one
-
- 123-35-3
7-methyl-3-methene-1,6-octadiene
Conditions
| Conditions |
Yield |
|
at 610 ℃;
|
|
-
- 106-23-0,26489-02-1
3,7-dimethyl-oct-6-enal
-
- 123-35-3
7-methyl-3-methene-1,6-octadiene
-
- 99-86-5
1-methyl-4-isopropyl-1,3-cyclohexadiene
-
- 586-67-4
3,8-p-Menthadien
-
- 491-07-6,1074-95-9,1196-31-2,3391-87-5,10458-14-7,14073-97-3,18309-28-9,36977-92-1,117773-78-1,89-80-5,17627-49-5,7786-64-3
(+/-)-menthone
Conditions
| Conditions |
Yield |
|
With aluminum oxide; carbon dioxide; In hexane; at 190 ℃; Supercritical conditions;
|
21.4%
6.6%
8.2%
6.9%
6.3% |
123-35-3 Upstream products
-
126-91-0
linalool
-
115-95-7
linalool acetate
-
13066-51-8
γ-geraniol
-
18172-67-3
beta-pinene
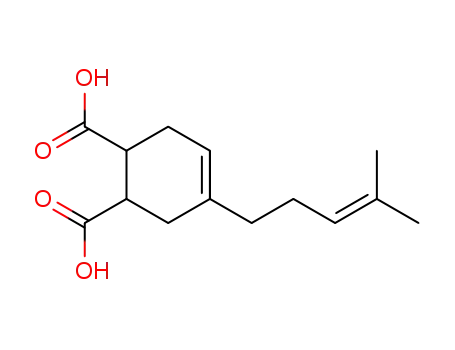
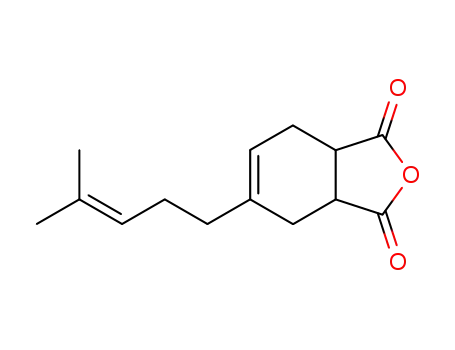
123-35-3 Downstream products
-
107821-52-3
4-(4-methyl-pent-3-enyl)-cyclohex-4-ene-1,2-dicarboxylic acid
-
29811-04-9
4-(4-methyl-3-pentenyl)-4-cyclohexene-1,2-dicarboxylic acid anhydride
-
29811-04-9
(+/-)-4-(4-methyl-pent-3-enyl)-cyclohex-4-ene-1r,2c-dicarboxylic acid-anhydride
-
29414-55-9
6,7-epoxymyrcene