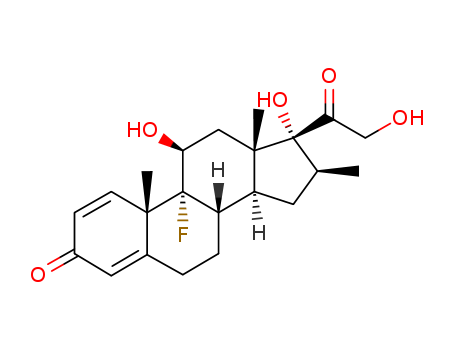
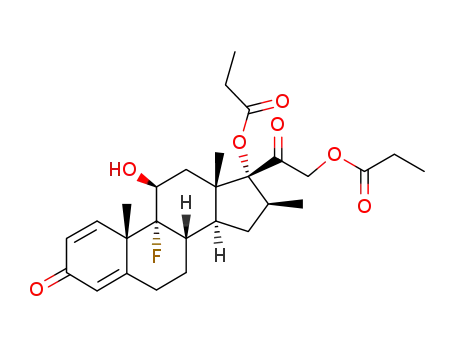
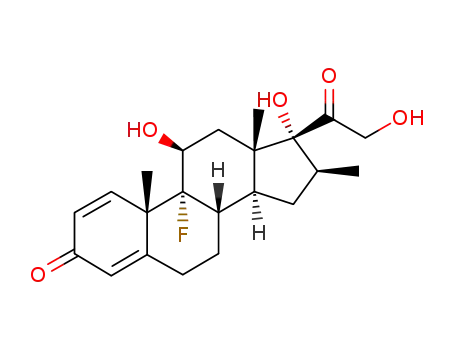
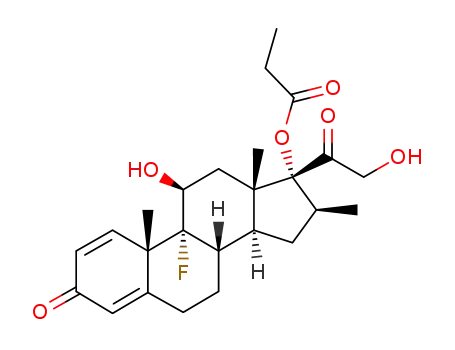
Top Quality Betamethasone 378-44-9 Hot Sell In Stock
- Molecular Formula:C22H29FO5
- Molecular Weight:392.468
- Appearance/Colour:white to off-white solid
- Vapor Pressure:2.81E-15mmHg at 25°C
- Melting Point:235-237 °C
- Refractive Index:118 ° (C=1, Dioxane)
- Boiling Point:568.2 °C at 760 mmHg
- PKA:12.13±0.70(Predicted)
- Flash Point:297.5 °C
- PSA:94.83000
- Density:1.32 g/cm3
- LogP:1.89570
Betamethasone(Cas 378-44-9) Usage
|
Description
|
Betamethasone is a steroid medication. |
|
Acute toxicity
|
Oral-mouse LD50:> 4500 mg/kg
|
|
Flammability and hazard characteristics
|
Combustible;Its combustion produces toxic fumes of fluoride.
|
|
Storage Characteristics
|
Ventilated, low-temperature ,dry storeroom.
|
|
Extinguishing agent
|
Dry powder , foam, sand, carbon dioxide, water spray.
|
|
Manufacturing Process
|
Betamethasone acetate is converted to betamethasone by means of hydrochloric acid in a methanol-chloroform-water mixture as described in US Patent 3,164,618.
|
|
Therapeutic Function
|
Glucocorticoid
|
|
Flammability and Explosibility
|
Nonflammable
|
|
Biochem/physiol Actions
|
Betamethasone, an?isomer?of dexamethasone is also termed as 9α-fluoro-16β-methyl-11 β,17,21-trihydroxypregna-1,4-dien-3,20-dione or 9α-fluoro-16β-methylprednisolone (27.1.52). It can be used as an anti-itch agent and treating dermatitis?and?eczema.
|
|
Side effects
|
Common Side Effects: Stinging, burning, itching, irritation, dryness, redness, stretch marks, skin thinning or discoloration, acne.
Serious Side Effects: Skin infections, severe rash, dizziness, trouble breathing. |
|
Safety Profile
|
Low toxicity by ingestion. Anexperimental teratogen. Other experimental reproductiveeffects. When heated to decomposition it emits toxicfumes of F-.
|
| Mechanism of Action |
Reduces inflammation by inhibiting the release of chemicals that cause swelling, redness, and itching in response to irritation or allergic reactions. |
|
Drug interactions
|
Potentially hazardous interactions with other drugs Aldesleukin: avoid concomitant use. Antibacterials: metabolism accelerated by rifampicin; metabolism possibly inhibited by erythromycin; concentration of isoniazid possibly reduced. Anticoagulants: efficacy of coumarins and phenindione may be altered. Antiepileptics: metabolism accelerated by carbamazepine, fosphenytoin, phenobarbital, phenytoin and primidone. Antifungals: increased risk of hypokalaemia with amphotericin - avoid; metabolism possibly inhibited by itraconazole and ketoconazole. Antivirals: concentration possibly increased by ritonavir. Ciclosporin: rare reports of convulsions in patients on ciclosporin and high-dose corticosteroids. Cobicistat: concentration of betamethasone possibly increased. Diuretics: enhanced hypokalaemic effects of acetazolamide, loop diuretics and thiazide diuretics. Vaccines: high dose corticosteroids can impair immune response to vaccines; avoid with live vaccines.
|
|
Metabolism
|
Corticosteroids are metabolised mainly in the liver but also in other tissues, and are excreted in the urine. The slower metabolism of the synthetic corticosteroids with their lower protein-binding affinity may account for their increased potency compared with the natural corticosteroids.
|
| Onset of Action |
For skin conditions: Improvement usually starts within a few days.
For conditions like bursitis, arthritis, and foot issues: Symptom relief often occurs within 3-4 days. |
|
Category
|
Toxic substances
|
|
Brand name
|
Celestone Syrup and Tablets (Schering).
|
InChI:InChI=1/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
378-44-9 Relevant articles
Antenatal Betamethasone for Women at Risk for Late Preterm Delivery
Cynthia Gyamfi-Bannerman, M.D., Elizabeth A. Thom, Ph.D., Sean C. Blackwell, M.D., Alan T.N. Tita, M.D., Ph.D., Uma M. Reddy, M.D., M.P.H., George R. Saade, M.D., Dwight J. Rouse, M.D.
, N Engl J Med 2016;374:1311-1320
Infants who are born at 34 to 36 weeks of gestation (late preterm) are at greater risk for adverse respiratory and other outcomes than those born at 37 weeks of gestation or later. It is not known whether betamethasone administered to women at risk for late preterm delivery decreases the risks of neonatal morbidities. Administration of betamethasone to women at risk for late preterm delivery significantly reduced the rate of neonatal respiratory complications.
The effects of betamethasone on clinical outcome of the late preterm neonates born between 34 and 36 weeks of gestation
Yas Arimi, Narges Zamani, Mamak Shariat & Hossein Dalili
, BMC Pregnancy and Childbirth volume 21, Article number: 774 (2021)
The neonates receiving betamethasone suffered more from respiratory distress syndrome (49% versus 31%, p = 0.008, RR = 1.59 95% CI (1.12–2.27)) and requiring more respiratory support (71% versus 50%, p = 0.002, RR = 1.43 95% CI (1.13–1.80)) as compared to the control group. There was no difference between the two groups in other neonatal adverse events or death.
A novel route for the preparation of betamethasone from 9α-hydroxyandrost-4-ene-3,17-dione (9αOH-AD) by chemical synthesis and fermentation
Tang, Jie,Liu, Xirong,Zeng, Chunlin,Meng, Hao,Tian, Mi,Guo, Cancheng
, p. 266 - 270 (2017/06/19)
A novel and efficient synthesis of betam...
378-44-9 Process route
-
- 5593-20-4
betamethasone dipropionate
-
- 5534-13-4
Betamethasone propionate
-
-
betamethasone 21-monopropionate
Conditions
| Conditions |
Yield |
|
With sulfuric acid; In acetonitrile; at 20 ℃; for 20h; Further Variations:; Temperatures; Product distribution;
|
|
-
- 5593-20-4
betamethasone dipropionate
-
- 5534-13-4
Betamethasone propionate
-
- 24703-00-2,24703-12-6,55879-47-5
6β-hydroxybetamethasone
-
- 78144-00-0,91677-33-7
6β-hydroxybetamethasone 17-propionate
Conditions
| Conditions |
Yield |
|
With phosphate buffer; air; plasma of 20 d pregnant Sprague-Dawley rat; at 37 ℃; for 1h; Product distribution; metabolism with tissues (plasma, liver, brain, placenta) from mothers and fetuses of Sprague-Dawley rats sacrificed on day 20 of pregnancy and mice on day 17 of pregnancy, further in vivo;
|
67.8 % Chromat.
2.3 % Chromat.
14.8 % Chromat.
1.4 % Chromat. |
378-44-9 Upstream products
-
5593-20-4
betamethasone dipropionate
-
987-24-6
betamethasone 21-acetate
-
884488-47-5
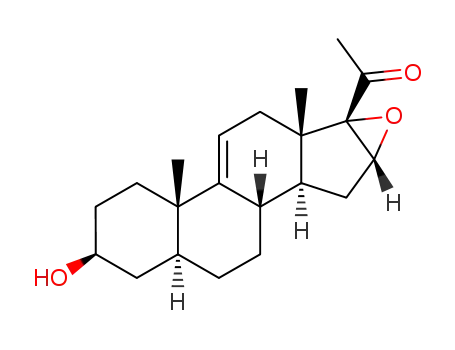
16α,17α-epoxy-3β-hydroxy-5α-pregn-9(11)-en-20-one
-
37413-99-3
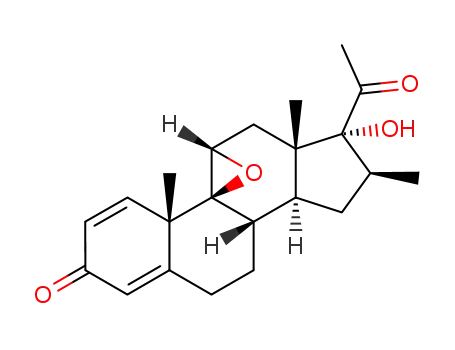
16β-methyl-9β,11β-epoxy-17α-hydroxy-1,4-pregnadiene-3,20-dione
378-44-9 Downstream products
-
37926-75-3
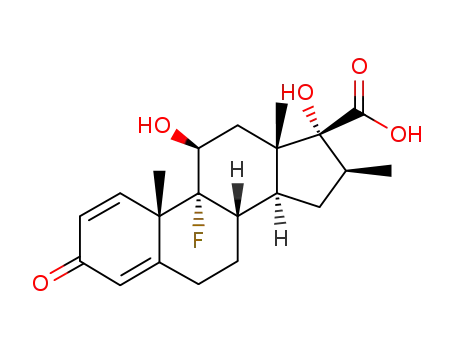
9α-fluoro-11β,17α-dihydroxy-16β-methyl-3-oxo-androsta-1,4-diene-17β-carboxylic acid
-
5534-13-4
Betamethasone propionate
-
5534-14-5
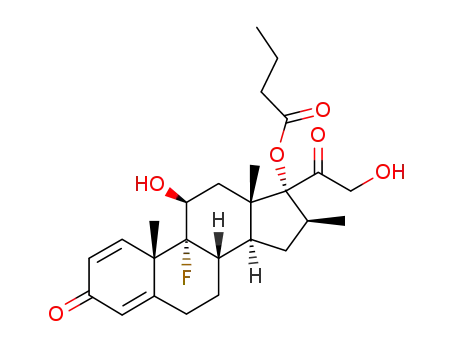
9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-1,4-pregnadiene-3,20-dione 17-butyrate
-
2152-44-5
betamethasone-valerate