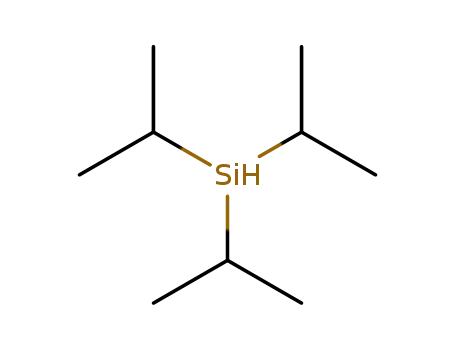
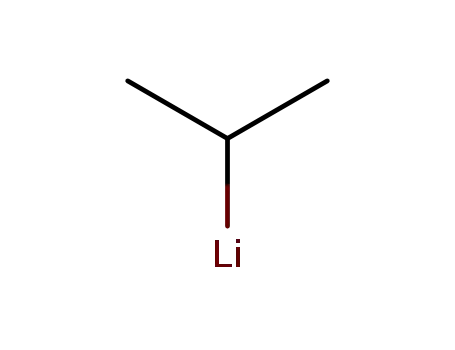Triisopropylsilyl chloride Good Supplier In Bulk Supply High Purity 13154-24-0
- Molecular Formula:C9H21ClSi
- Molecular Weight:192.804
- Appearance/Colour:Clear colorless liquid
- Vapor Pressure:1.16 mm Hg ( 20 °C)
- Refractive Index:n20/D 1.452(lit.)
- Boiling Point:199.1 ºC at 760 mmHg
- Flash Point:62.8 ºC
- PSA:0.00000
- Density:0.855 g/cm3
- LogP:4.40070
Triisopropylsilyl chloride(Cas 13154-24-0) Usage
|
Preparation
|
Method 1: Triisopropyl silane reacts with hydrochloric acid to chlorinate the hydrogen on silicon.
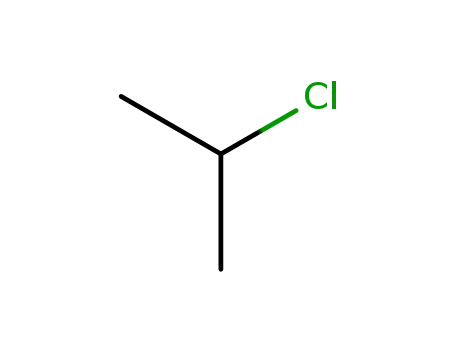
Method 2: Silicon tetrachloride reacts with isopropyl lithium to form triisopropylchlorosilane. |
|
Physical properties
|
bp 198 °C/739 mmHg; d 0.901 g cm?3.
|
|
Application
|
Triisopropylsilyl chloride is used as a basic intermediate in the synthesis of organosilicon materials. Functions as a blocking agent for silicone oils or silicone rubbers. |
| Hazards |
Triisopropylsilyl chloride is corrosive, causing severe skin burns and eye damage, and reacts with water to produce toxic hydrogen chloride gas. |
| Supplier |
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. |
InChI:InChI=1/C9H21ClSi/c1-7(2)11(10,8(3)4)9(5)6/h7-9H,1-6H3
Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
13154-24-0 Relevant articles
Efficient and Practical Procedure for the Esterification of the Free α-Carboxylic Acid of Amino Acid Residues with β-(Trimethylsilyl)ethoxymethyl Chloride and Triisopropylsilyl Chloride
Jean-Simon Suppo , Danilo Pereira de Sant’Ana , Luiz Carlos Dias , Renata Marcia de Figueiredo* , Jean-Marc Campagne*
, Synthesis 2014; 46(22): 3075-3084
An efficient and practical procedure for the free α-carboxylic acid esterification of amino acid residues with β-(trimethylsilyl)ethoxymethyl chloride and triisopropylsilyl chloride is described. The reaction takes place under mild conditions and the expected protected amino acids are obtained in good to excellent yields. Our method provides a useful alternative for the C-terminal carboxylic acid protection of amino acids and peptides. Moreover, the removal of such protection was also achieved under mild conditions, without affecting either the other protecting groups at the α-amino moiety and side chains or the optical integrity at the α-position of the amino acid residues.
Hexachloroethane: a highly efficient reagent for the synthesis of chlorosilanes from hydrosilanes
Pongkittiphan, Veerachai,Theodorakis, Emmanuel A.,Chavasiri, Warinthorn
, p. 5080 - 5082 (2009)
A new and efficient chlorination protoco...
EFFECTIVE SILYLATION OF CARBOXYLIC ACIDS UNDER SOLVENT-FREE CONDITIONS WITH tert-BUTYLDIMETHYLSILYL CHLORIDE (TBDMSCL) AND TRIISOPROPYLSILYL CHLORIDE (TIPSCL)
Habib Firouzabadi,Naser Iranpoor &Hamid Reza Shaterian
, Phosphorus, Sulfur, and Silicon and the Related Elements, Volume 166, 2000 - Issue 1
Various types of carboxylic acids can be converted effectively to their corresponding TBDMS and TIPS esters using TBDMSCI and TIPSCI in the presence of imidazole under solvent-free conditions. The advantage of this modified method in comparison with that reported by Corey is the elimination of DMF, which eliminates aqueous work-up.
13154-24-0 Process route
-
- 33974-42-4
tri(iso-propyl)methoxysilane
-
- 13154-24-0
triisopropylsilyl chloride
Conditions
| Conditions |
Yield |
|
With iron(III) chloride; acetyl chloride; In 1,2-dichloro-ethane; for 24h;
|
88% |
|
With hydrogenchloride; In water; at 20 ℃; for 10h;
|
60% |
|
With hydrogenchloride; at 24 ℃; for 2h;
|
98 % Chromat. |
|
Multi-step reaction with 2 steps
1: 100 percent / aq. HCl / tetrahydrofuran / 65 °C
2: 95 percent / aq. HCl / 2 h / 24 °C
With hydrogenchloride; In tetrahydrofuran;
|
|
-
- 75031-67-3
1-(triisopropylsilyl)oxybutane
-
- 13154-24-0
triisopropylsilyl chloride
Conditions
| Conditions |
Yield |
|
With hydrogenchloride; In water; at 20 ℃; for 10h;
|
89% |
|
With hydrogenchloride; In tetrahydrofuran; at 24 ℃; for 4h;
|
95 % Chromat. |
13154-24-0 Upstream products
-
1888-75-1
isopropyllithium
-
75-29-6
isopropyl chloride
-
6485-79-6
chlorotriisopropylsilane
-
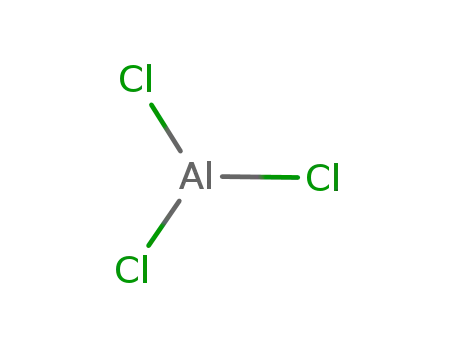
7446-70-0
aluminium trichloride
13154-24-0 Downstream products
-
17903-14-9
triisopropyl(phenyl)silane
-
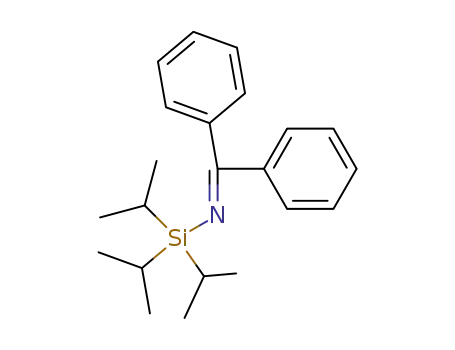
17599-30-3
N-Triisopropylsilyl-benzophenonimin
-
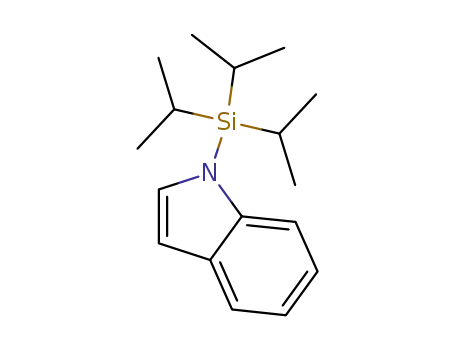
123191-00-4
1-(triisopropylsilyl)-1H-indole
-
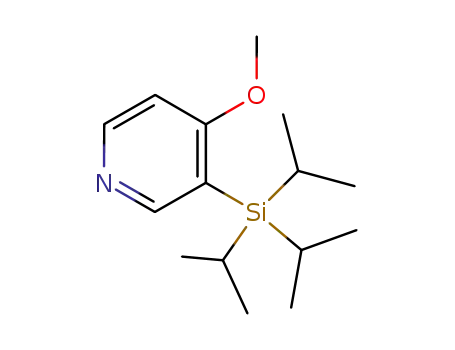
126378-42-5
4-methoxy-3-(trisopropylsilyl)pyridine