China cas 4394-00-7 manufacturer wholesale Niflumic acid at affordable price
- Molecular Formula:C13H9F3N2O2
- Molecular Weight:282.222
- Appearance/Colour:Pale yellow crystalline powder
- Vapor Pressure:2.18E-06mmHg at 25°C
- Melting Point:203-204 °C
- Boiling Point:378 °C at 760 mmHg
- PKA:pKa 2.26 ± 0.08;4.44± 0.03(H2O,t =25±0.1,I=0.01(NaCl))(Approximate)
- Flash Point:182.4 °C
- PSA:62.22000
- Density:1.449 g/cm3
- LogP:3.61520
Niflumic acid(Cas 4394-00-7) Usage
|
Manufacturing Process
|
Niflumic acid is prepared as follows: Nicotinic acid, m-trifluoromethylaniline, and potassium iodide are intimately mixed and heated on an oil bath at 140°C. The mixture melts to give a dark red liquid. The temperature of the oil bath is allowed to fall to 100°C and is maintained at this temperature for an hour and a half. The mixture puffs up and forms a yellow crystalline mass. After cooling to ordinary temperature, this mass is ground up in a mortar and extracted several times with small volumes of ether to remove excess m�trifluoromethylaniline. The residue is then washed twice with 10 ml of distilled water to remove m-trifluoromethylaniline hydrochloride and potassium iodide, and finally twice with 10 ml of 95% alcohol to remove colored resinous contaminants. After drying at 100°C, 2-(m-trifluoromethylanilino)nicotinic acid is obtained as pale yellow needles (from 70% ethanol) melting at 204°C (Kofler block).
|
|
Therapeutic Function
|
Antiinflammatory
|
|
Biochem/physiol Actions
|
Niflumic acid (NFA), a γ-aminobutyric acid type A receptor (GABAARs) antagonist is a non-steroidal anti-inflammatory drug (NSAID) and it belongs to the fenamate class. It is also a blocker of chloride ion channel and a calcium-activated chloride channel (CaCC) inhibitor. NFA possesses anti-inflammatory property and is useful in treating rheumatic disorders. It is also an inhibitor of N-methyl-D-aspartate receptor and glycine receptor. NFA also inhibits enzymes associated with the prostaglandins synthesis.
|
|
Synthesis
|
Niflumic acid, 2-3-(trifluoromethyl)anilino nicotinic acid (3.2.21), is syn�thesized either by the reaction of 2-chloronicotinic acid with 3-trifluoromethylaniline [84–86], or 2-aminonicotinic acid with 1-bromo-3-trifluoromethylbenzene [87].
|
InChI:InChI=1/C13H9F3N2O2/c14-13(15,16)8-3-1-4-9(7-8)18-11-10(12(19)20)5-2-6-17-11/h1-7H,(H,17,18)(H,19,20)/p-1
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success. Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
4394-00-7 Relevant articles
1,2-dihydro-3,1-benzoxazin-4-one and 4-H-1,2-dihydro-pyrido-[2,3-d]-[1,3]-oxazin-4-one derivatives as potential prodrugs. Part II: Hydrolysis
Schwenker,Chen
, p. 887 - 890 (1991)
-
Development of LM98, a Small-Molecule TEAD Inhibitor Derived from Flufenamic Acid
Mélin, Léa,Abdullayev, Shuay,Fnaiche, Ahmed,Vu, Victoria,González Suárez, Narjara,Zeng, Hong,Szewczyk, Magdalena M.,Li, Fengling,Senisterra, Guillermo,Allali-Hassani, Abdellah,Chau, Irene,Dong, Aiping,Woo, Simon,Annabi, Borhane,Halabelian, Levon,LaPlante, Steven R.,Vedadi, Masoud,Barsyte-Lovejoy, Dalia,Santhakumar, Vijayaratnam,Gagnon, Alexandre
, p. 2982 - 3002 (2021/08/03)
The YAP-TEAD transcriptional complex is ...
Design and synthesis of niflumic acid-based N-acylhydrazone derivatives as novel anti-inflammatory and analgesic agents
Kheradmand, Amin,Navidpour, Latifeh,Shafaroodi, Hamed,Saeedi-Motahar, Ghazaleh,Shafiee, Abbas
, p. 2411 - 2420 (2013/07/26)
A new series of niflumic acid-based N-ac...
Synthesis, stability studies, anti-inflammatory activity and ulcerogenicity of morpholinoalkyl ester prodrugs of niflumic acid
Talath, Sirajunisa,Gadad, Andanappa K.
, p. 744 - 752 (2007/10/03)
In search for potential prodrugs for ant...
Stability studies of some glycolamide ester prodrugs of niflumic acid in aqueous buffers and human plasma by HPLC with UV detection
Talath, Sirajunisa,Shirote, Pramod J.,Lough, W. John,Gadad, Andanappa K.
, p. 631 - 639 (2008/02/12)
Glycolamide esters (compounds 1-17) of 2...
4394-00-7 Process route
-
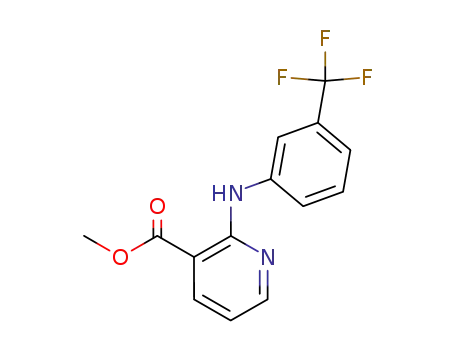
- 59361-45-4
methyl 2-((3-(trifluoromethyl)phenyl)amino)nicotinate
Conditions
| Conditions |
Yield |
|
With water; sodium hydroxide; In methanol; at 80 ℃;
|
82% |
-
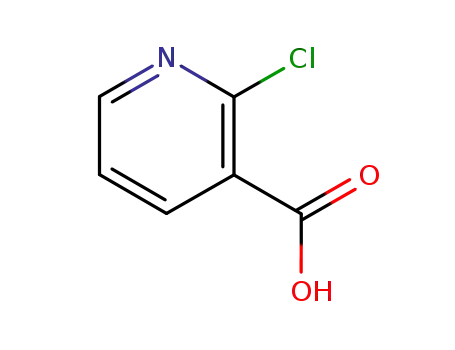
- 2942-59-8
2-chloronicotinic acid
-
- 98-16-8
3-trifluoromethylaniline
Conditions
| Conditions |
Yield |
|
With pyridine; toluene-4-sulfonic acid; In water; Heating;
|
|
|
With potassium iodide; at 100 - 140 ℃; for 1.5h;
|
|
4394-00-7 Upstream products
-
2942-59-8
2-chloronicotinic acid
-
98-16-8
3-trifluoromethylaniline
-
137488-50-7
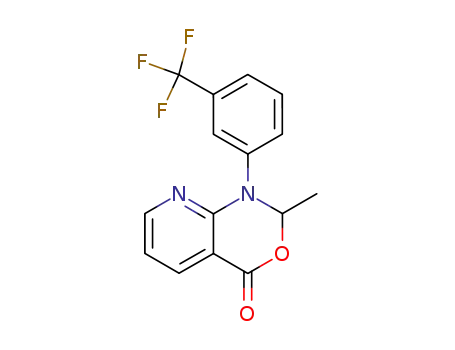
1-(3'-trifluoromethylphenyl)-2-methyl-4H-1,2-dihydro-pyrido-<2,3-d>-<1,3>-oxazin-4-one
-
137488-34-7
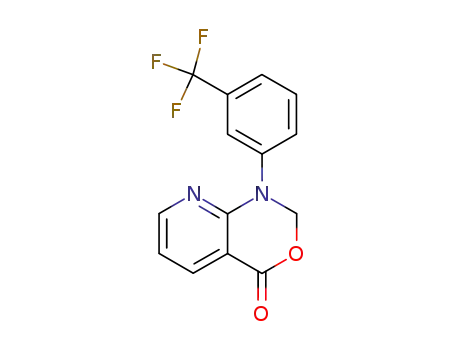
1-(3'-trifluoromethylphenyl)-4H-1,2-dihydro-pyrido-<2,3-d>-<1,3>-oxazin-4-one
4394-00-7 Downstream products
-
66898-62-2
talniflumate
-
137488-34-7
1-(3'-trifluoromethylphenyl)-4H-1,2-dihydro-pyrido-<2,3-d>-<1,3>-oxazin-4-one
-
66898-68-8
2-<<3-(trifluormetil)fenil>amino>-3-piridincarboxilato de pivaloiloximetilo
-
137488-50-7
1-(3'-trifluoromethylphenyl)-2-methyl-4H-1,2-dihydro-pyrido-<2,3-d>-<1,3>-oxazin-4-one