Factory sells Beta-cyclodextrin 7585-39-9 with sufficient production capacity
- Molecular Formula:C42H70O35
- Molecular Weight:1135
- Appearance/Colour:white powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:298-300 °C
- Refractive Index:1.7500 (estimate)
- Boiling Point:1578.5oC at 760 mmHg
- PKA:11.73±0.70(Predicted)
- Flash Point:908.5oC
- PSA:554.05000
- Density:1.624 g/cm3
- LogP:-15.23060
Hebei KuiSheng Trading Co., LTD ,a company specializing in the production and supply of chemicals for various industries. we take pride in our ability to carefully formulate chemicals that meet the highest standards of quality, efficiency, and safety. Through advanced technology and strict quality control measures, we ensure that our products consistently deliver exceptional performance and reliability. Whether you are in need of chemicals for pharmaceutical, agricultural, or industrial applications, we offer a wide range of solutions to meet your specific requirements. Our team is dedicated to providing excellent customer service and we strongly believe in establishing long-lasting business relationships built on trust and mutual success.
Beta-cyclodextrin(Cas 7585-39-9) Usage
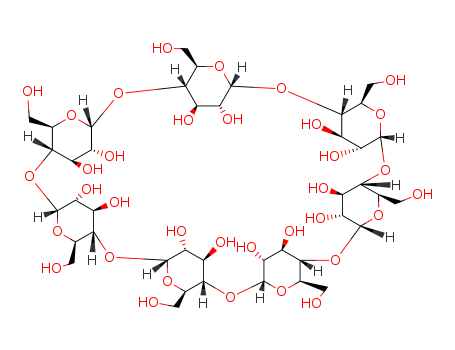
| Structure: |
Cyclic oligosaccharide composed of seven glucose units linked by α-(1,4) glycosidic bonds. Cone-shaped with a hydrophobic inner cavity and a hydrophilic exterior. |
| Source: |
Beta-cyclodextrin is derived from the breakdown of natural polymers, typically cellulose or starch. Commercially produced through Bacillus macerans or B. circulans fermentation of starch. |
| Safety: |
Generally safe for oral use but nephrotoxic when administered parenterally (due to accumulation in kidneys). Not metabolized parenterally but broken down by colon microflora when ingested. Approved for use in food and pharmaceuticals in many countries. |
| Uses |
Beta-Cyclodextrin enhances solubility, stability, and bioavailability of drugs by forming inclusion complexes. Commonly used in tablet and capsule formulations, especially for direct compression (requires lubricant).
|
|
Flammability and Explosibility
|
Nonflammable
|
| Acute Toxicity (LD50): |
Mouse (IP): 0.33 g/kg; Rat (IP): 0.36 g/kg; Rat (IV): 1.0 g/kg; Rat (oral): 18.8 g/kg |
InChI:InChI=1/C42H70O35.H2O/c43-1-8-29-15(50)22(57)36(64-8)72-30-9(2-44)66-38(24(59)17(30)52)74-32-11(4-46)68-40(26(61)19(32)54)76-34-13(6-48)70-42(28(63)21(34)56)77-35-14(7-49)69-41(27(62)20(35)55)75-33-12(5-47)67-39(25(60)18(33)53)73-31-10(3-45)65-37(71-29)23(58)16(31)51;/h8-63H,1-7H2;1H2
Allow me to outline some of our key advantages: 1. High quality with competitive prices: We strive to offer chemicals of the highest quality while remaining competitive in the market. 2. All purity >99%: Our products undergo rigorous purification processes to guarantee high purity levels. 3. Manufacturer with factory prices: As a manufacturer, we have the ability to offer high-quality products at competitive factory prices. 4. Fast and safe delivery: We understand the importance of timely and secure deliveries. Therefore, we have established reliable logistics networks to ensure efficient transportation of our products. 5. OEM is welcome: We are open to providing Original Equipment Manufacturer (OEM) services, allowing you to customize products according to your specific needs. 6. Sufficient stock: Our extensive inventory ensures that we can fulfill orders promptly, regardless of their size or complexity. We are confident that with our extensive experience and dedication to excellence, we can be your trusted partner in chemical solutions. We would greatly appreciate the opportunity to work with you and contribute to the success of your business. Should you require any further information or have specific inquiries, please do not hesitate to contact us. We look forward to hearing from you.
7585-39-9 Relevant articles
Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component
Romina L. Abarca , Francisco J. Rodríguez , Abel Guarda , María J. Galotto , Julio E. Bruna
, Food Chemistry Volume 196, 1 April 2016, Pages 968-975
A higher EE was obtained (34.8%) for the inclusion complex 1:0.5 than for other molar rates. Both DSC and TGA of the inclusion complexes showed the presence of endothermic peaks between 80 °C and 150 °C, attributed to a complexation phenomenon. Antimicrobial tests for mycelial growth reduction under atmospheric conditions proved the fungistatic behaviour of the inclusion complexes against Botrytis cinerea.
Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers
Giuseppe Tripodo † , Christian Wischke , Axel T. Neffe , Andreas Lendlein
, Carbohydrate Research Volume 381, 15 November 2013, Pages 59-63
As characterized by FTIR, mass spectrometry, elemental analysis, and NMR, mono-Ts-βCD was obtained with a molar purity of >98 mol %. From mono-Ts-βCD, β-cyclodextrin dimers linked by ethylenediamine (bis-Et-βCD) were successfully prepared (yield 93%, purity 96 mol %) in a one-step approach using an anion exchange resin to trap leaving groups that typically interfere in the reaction. This synthesis procedure with a direct collection of side-products may be a general strategy applicable for nucleophilic substitution of tosylated cyclodextrins.